
Your Unified Solution
Labguru integrates an electronic lab notebook, LIMS, inventory management, automation, and informatics from different sources into one easy-to-use platform. It’s the ultimate laboratory management software and data management solution.
Centralize your data in one format within secure, cloud-based software. The lab management software streamlines lab operations, prevents data loss, and speeds up your research outcomes – saving you time and money.
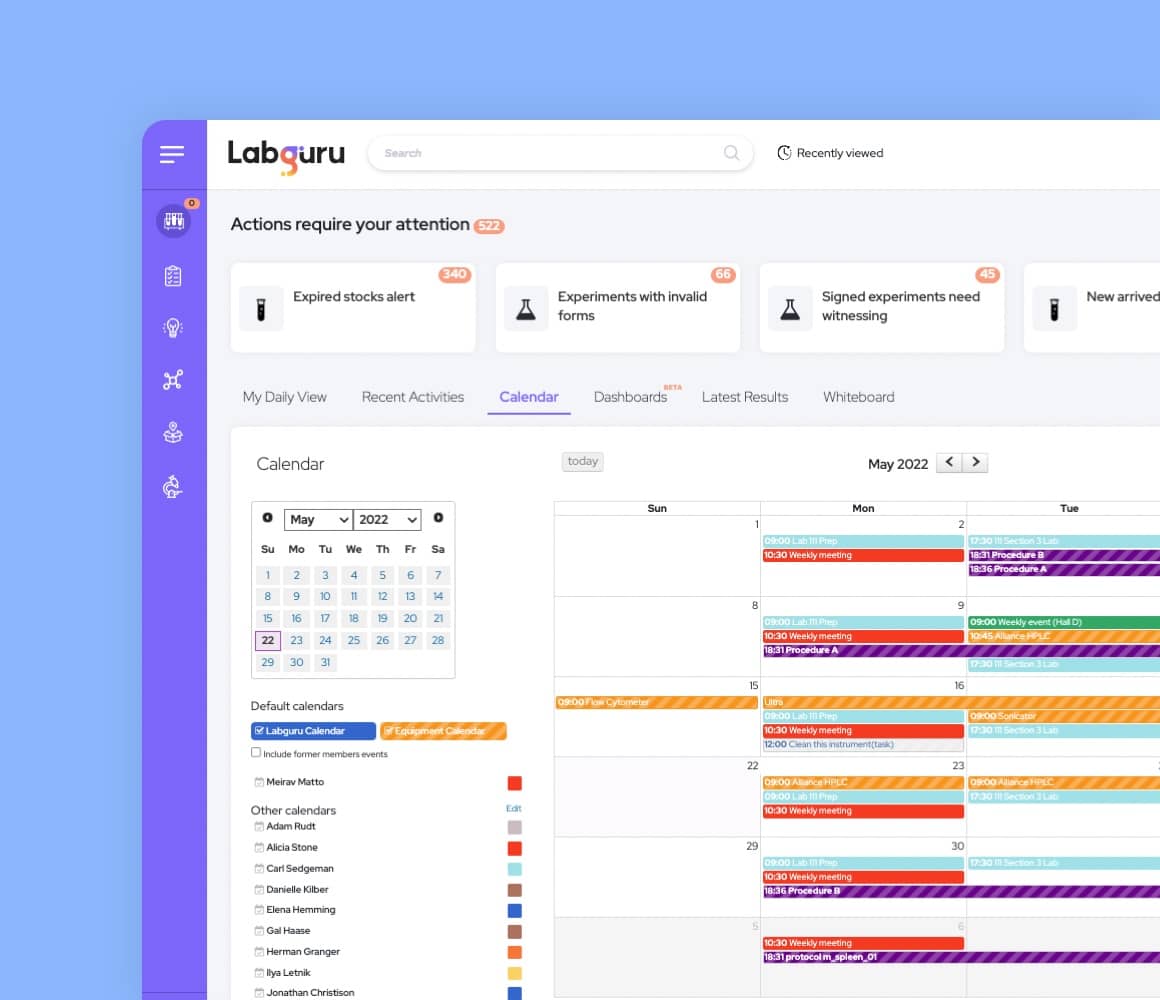
Electronic Lab Notebook
Run your lab efficiently and maximize your research output with our digital lab notebook.
Our ELN software lets you plan and document experiments, track progress, standardize lab processes, and share results.
-
Easy project & lab data management
-
Secure data hosting & regulation compliance
-
Integrated inventory & sample management
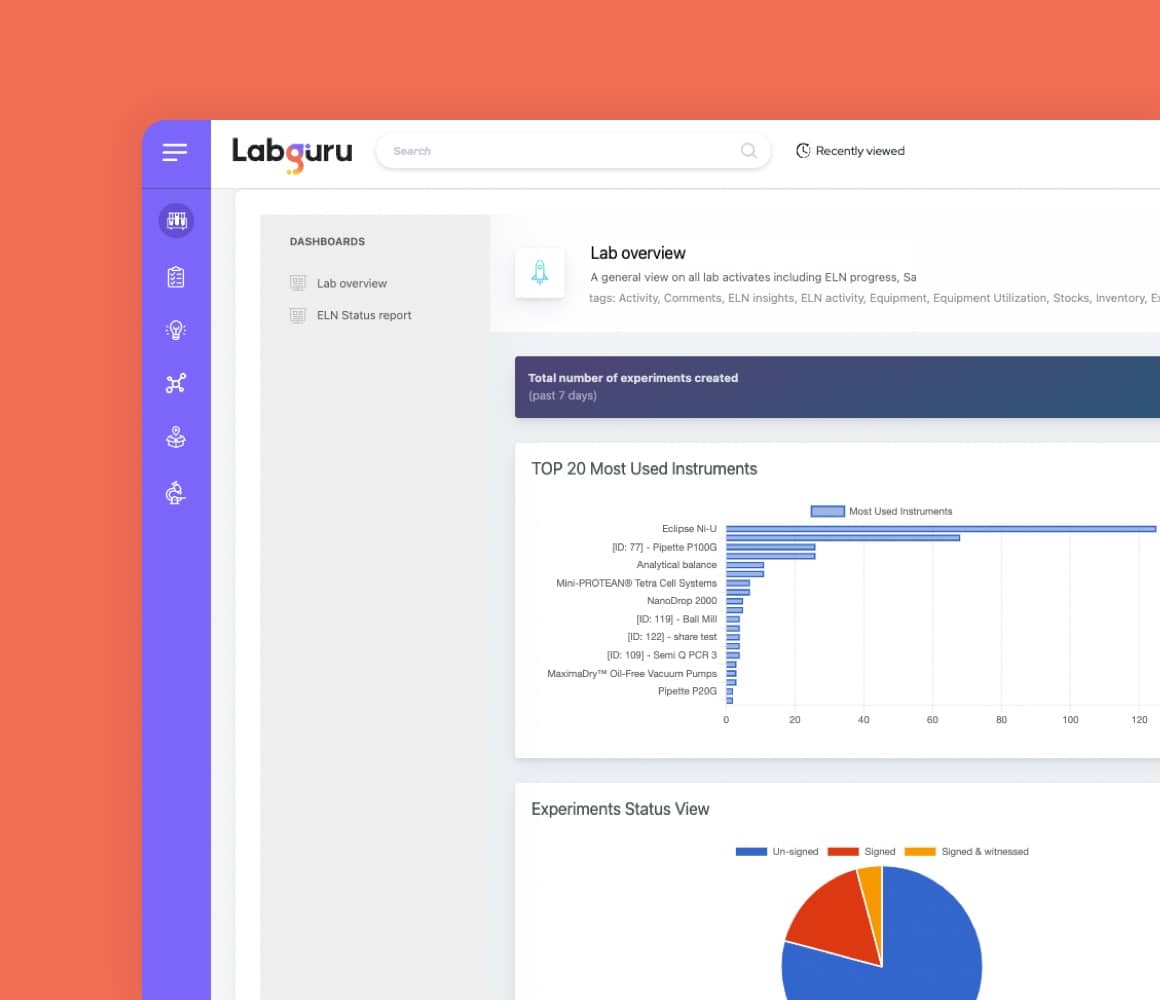
Laboratory Information Management System
The lab management software enhances sample tracking, storage, inventory management, equipment scheduling, maintenance, and more.
Conduct certification analysis, create visualized reports, and share data with your lab members.
-
Automate processes & create workflows
-
Increase productivity & reduce human error
-
Ensure data integrity & prevent data loss
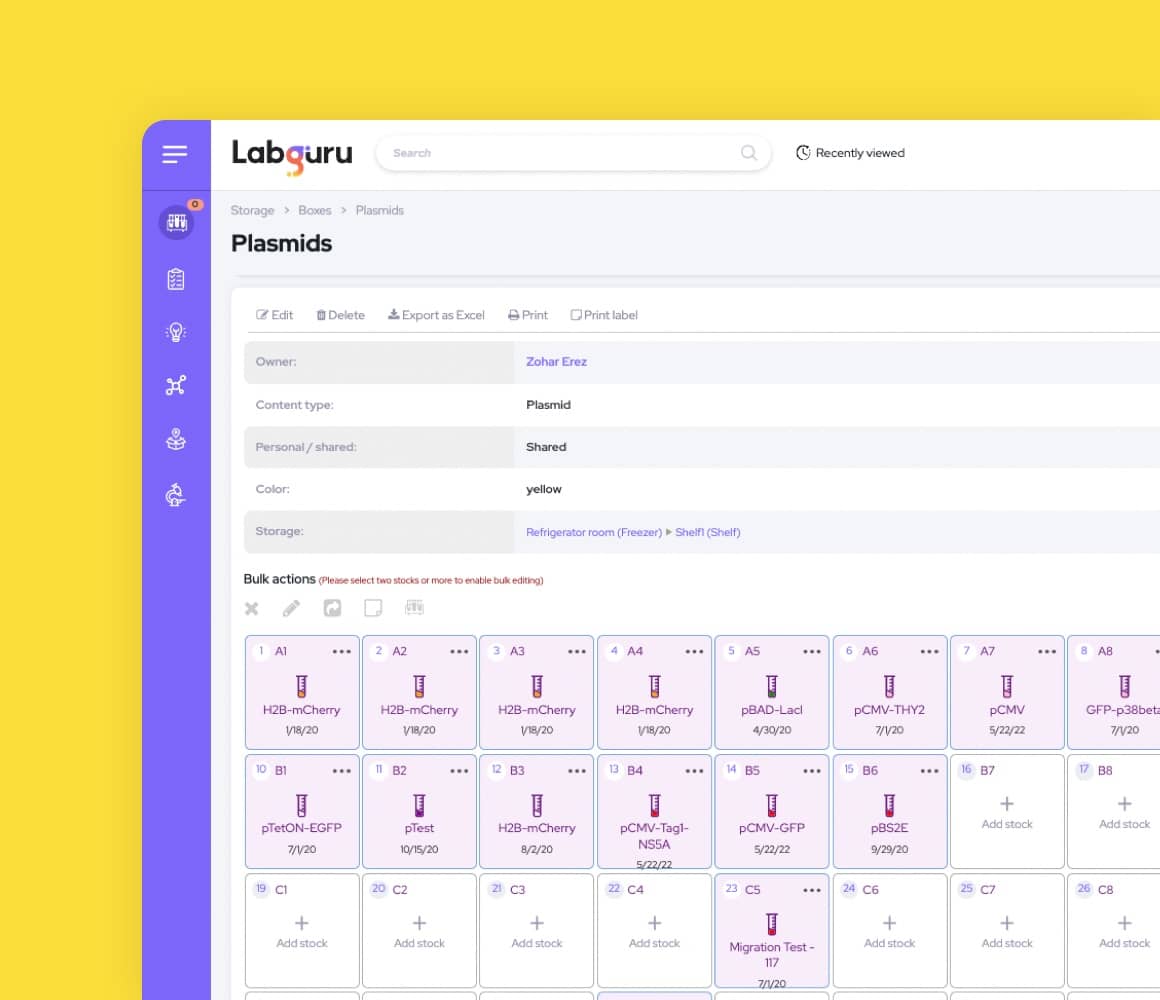
Integrated Inventory Management
The lab management system centralizes ordering processes to eliminate duplicate orders and maps locations to facilitate searches for materials and samples.
-
Keep lab costs under control
-
Track lab inventories and receive notifications when running low on items
-
Manage large collections of living specimens, tissues and samples
Best-in-Class Automation Solutions
Our lab management system allows you to automate any laboratory management or data processing routine with ease. Save time and eliminate human error with customized step-by-step workflows.
-
Set triggers and rules to define your workflows and automation processes.
-
Build tailored workflows and assemble predefined steps to design customized processes.
-
Add code with the Lab Scripter.
.png?width=1392&height=1200&name=Frames%20for%20website%20(5).png)
Additional Key Components
Do More Science With Labguru
75%
reported increased productivity
600+
companies
50%
of new customers come from word of mouth
Streamline Research with Labguru
Integrated Inventory Management
Enhance inventory management, keep track of storage locations, and optimize orders.
Remote & Collaborative Lab Work
Access research data safely anytime, anywhere. Work from home effectively and enhance collaboration.
Automation
Automate repetitive tasks for optimal performance. Easily design customized workflows to save time.
Highly Customizable Platform
Our flexible architecture provides support for a wide range of customers and customer needs.
Professional Support
Our team of PhD application scientists are always available.
Fast & Simple Onboarding
Transition quickly and seamlessly with zero data loss for instant productivity.
Security
Store your data safely in the cloud in SOC-compliant data centers by Amazon Web Services.
Regulation and Standards
Sign and witness experiments in full compliance with FDA CFR 21 part 11 regulations.
Full Data Ownership
You own all your data, always. Never worry about being locked out or denied access to your intellectual property.
Official Partner

What Our Customers Say
"I can confidently say that Labguru has saved us tens of thousands of dollars. I really enjoy Labguru. I feel like I have a high bar for recommending things, so it means a lot when I say that I’m honestly pleased with it."

Dr. Derek Croote
CTO at IgGenix
"Labguru saves us 40% of our working hours every month because its consistency and powerful search function eliminates the need to dig through notebooks, Google Drive folders, and physical storage rooms."

Dr. Yasmin Chau
Principal Scientist at Double Rainbow
"Labguru simply performed better than the other systems we looked at. We specifically wanted a system with good workflow capabilities that could support our ways of working... It was an easy decision to go with Labguru and we haven’t been disappointed"

Colin Pashia
Metallurgical and Materials Engineering Lead at Dodge Industrial
"With Labguru, we save 75% of the time previously required to manage and update our lab notebooks."

Dino Ossola
Senior Group Leader at Cerevance
"We chose Labguru because it enabled us to manage all daily activities, including conducting experiments and tracking inventory, in one place."

Dr. Chunyan Gu-Trantien
Project Director at Equaly

.png?width=150&height=61&name=Dodge_logo1%20(1).png)





%20(1).png?width=553&height=260&name=lab-room%20(2)%20(1).png)